How to Calculate Neutron Number
X is the relative abundance. Notice that your weight is different on the different worlds.

Counting Atoms Protons Neutrons Electrons Worksheet Counting Atoms Text Features Worksheet Worksheets
When it comes to atomic comparisons the neutrons are complementary to the latter via different scattering cross sections their susceptibility to magnetism their energy spectrum for inelastic.
. Warm cold and hot neutron radiation has a very important application in neutron scattering facilities where the radiation is also used in condensed matter research with the help of X-rays. When we calculate the theoretical mass of a nucleus using the masses of protons and neutrons and compare it to the experimental mass we observe that there is a small but relevant difference in the two masses. At this point we have to distinguish between two basic types of nuclear or neutron cross-sections.
In the case of a negative ion there are fewer protons than electrons. Example Heat Loss through a Window. The nucleus of protium 11H or simply hydrogen consists no neutron but only proton deuterium 12H has a neutron in the nucleus and tritium13H has two neutrons in the.
Isotopes are atoms of the same element that have different number of neutrons. D 2 x r Zr1 93 mm to calculate the hydraulic diameter the pitch of fuel pins is. Binding Energy formula is defined as the amount of energy required to separate a particle from a system of particles or to disperse all the particles of the system is calculated using Binding Energy Atomic Number Mass-pMass Number-Atomic Number Mass-n-Mass of atomc2To calculate Binding Energy you need Atomic Number Z Mass Number A.
To calculate the Reynolds number we have to know. μ 00000859 Nsm 2. A major source of heat loss from a house is through the windows.
Not all proto-neutron stars have the same mass and they have not finished contracting and the infalling matter is turbulent and not. Add the charge to the atomic number for negative ions. F 1 T.
Frequency is the number of completed wave cycles per second. In nuclear engineering a neutron moderator is a medium that reduces the speed of fast neutrons ideally without capturing any leaving them as thermal neutrons with only minimal thermal kinetic energyThese thermal neutrons are immensely more susceptible than fast neutrons to propagate a nuclear chain reaction of uranium-235 or other fissile isotope by colliding with. F denotes frequency and T stands for the time it takes to complete one wave cycle measured in seconds.
GRB 171205A is a low-luminosity long-duration gamma-ray burst GRB associated with SN 2017iuk a broad-line type Ic supernova SN. Notice that the weights on other worlds will automatically fill in. Write your answers in scientific notation containing 5 significant figures.
However this is not exact. This frequency definition leads us to the simplest frequency formula. Express your answers in joules.
When an ion has a negative charge the atom has gained electrons. Calculate the work done when 754 mole of an ideal gas is expanded reversibly and isothermally from 50114 to 162924 atm at 2500 oC. Suppose you have a chlorine 35 and 37.
Calculate the rate of heat flux through a glass window 15 m x 10 m in area and 30 mm thick if the temperatures at the inner and outer surfaces are 140C and 130C respectivelyCalculate the heat flux through this window. There might be zero one two more neutrons in the nuclei depending on the element. ENTER YOUR WEIGHT HERE The Planets MERCURY Your weight is.
You can click on the images of the planets to get more information about them from Bill Arnetts incredible Nine Planets web site. Microscopic Cross-sectionThe effective target area in m 2 presented by a single nucleus to an incident neutron beam is denoted the microscopic cross section σThe microscopic cross-sections characterize interactions with single isotopes and are a part of. A modification in the number of neutrons in the nucleus indicates an isotope.
Hydrogen has three isotopes. VENUS Your weight is. P 13 mm to calculate the hydraulic diameter the dynamic viscosity of saturated water at 300C is.
ME is the atomic mass of the element from the periodic table. M1 is the mass of one isotope. Velocity of light c 2998108 ms 1 u 16605410-27 kg Particle or atom Mass u proton 100728 neutron 100866 electron 000055 84Be 800531 2814Su 2797693 23692U 23504392 a 84Be Number of protons 4 Total mass of protons.
It is consistent with being formed in the core-collapse of a single CO star or in. The SI frequency unit is Hertz. Scroll down the page for examples and solutions.
This is a result from the binding energy which is responsible for binding protons and neutrons together in the nucleus of an atom. Heat loss through windows. Calculation of the Reynolds number.
In other words frequency tells us how many wave crests pass a given point in a second. The following diagrams show the isotopes of chlorine and how to calculate the relative atomic mass. Calculate the work done when 754 mole of an ideal gas is expanded reversibly and isothermally from 50114 to 162924 atm at.
M2 is the mass of the second isotope. The outer diameter of the cladding is. STP most commonly is used when performing calculations on gases such as gas density is calculated using STP Volume of Gas 273 Temperature of GasPressure of Gas 100To calculate STP you need Volume of Gas V Temperature of Gas T g Pressure of Gas PWith our tool you.
To calculate the total number of present electrons you simply add the amount of extra charge to the atomic number. Hypernova and Newborn Neutron Star. Click on the Calculate button.
STP in chemistry is the abbreviation for Standard Temperature and Pressure. Matter infalling onto a proto-neutron star in a core collapse supernova is falling in at approximately the escape velocity of a neutron star - for a 1 solar mass 12 km neutron star this is about 50 the speed of light. Isotopes are variants of a particular chemical element which differ in neutron number and consequently in nucleon number.
In order for them to be atoms of the same element their number of protons would be the same Examples. Formula to calculate relative abundance.

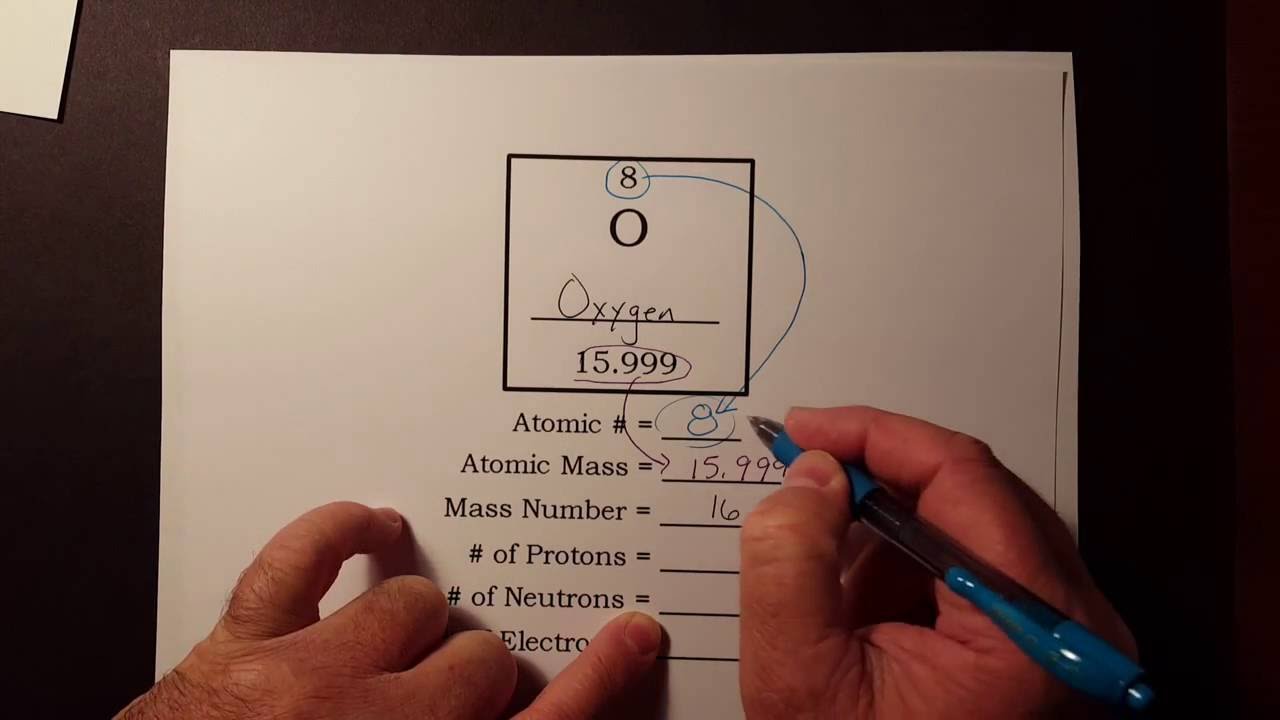
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Protons Proton Neutron Electron

Definition Of Mass Number With Examples Isoptopes Mass Number Definition Of Mass Atomic Mass Unit

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets

Protons Neutrons And Electrons Practice Worksheet Proton Neutron Electron Protons Practices Worksheets
Comments
Post a Comment